Join a Study
Sign Up Form
800 S Saginaw St., Flint, Michigan, 48507
5111 Auto Club Dr.,Suite 101, Dearborn, Michigan 48126
2525 Michigan Ave. , Chicago, IL 60616
Flint Main Location
Address: 800 S Saginaw St., Flint, Michigan, 48507
Phone: 810-243-0432
Email: contact.irimichigan@iinn.com
Chicago Location
Address: 2525 Michigan Ave. , Chicago, IL 60616
Phone: 312-567-8775
Email: contact.irichicago@iinn.com
Dearborn Location
Address: 5111 Auto Club Dr.,Suite 101, Dearborn, Michigan 48126
Phone: 810-243-0432
Email: contact.irimichigan@iinn.com
Frequently Asked Questions

What is a Clinical Trial?
Why should I participate in a clinical trial?
-
Join a study to help advance medicine through participation in a vaccine or therapeutic clinical trial.
-
There is no cost to participate,
-
No insurance required
-
You may receive compensation for study-related time and travel (amounts differ for each study)
-
Get all medical procedures done at NO cost, including labs and imaging (x rays, CT scans, EKGs, MRIs etc).
-
You can learn about new medications in development
What are the different phases of a Clinical trial?
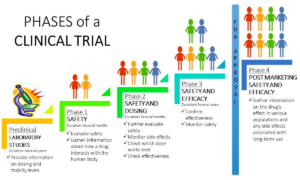
What is a Clinical Trial Participation Process?
Joining
To inquire about a clinical trial, please use the study finder page or call the contact number listed on the study advertisement or notice. A member of our staff will be able to answer questions and direct you to an appropriate study. Alternatively, if you meet the criteria for a study, a member of our team may reach out to you during a scheduled visit at Insight’s clinics.
Pre-Screening
A member of our research staff will explain the study briefly and ask some questions to determine whether a particular study is right for you. At this time, you can also ask about any questions you may have about the study.
Screening
Once it has been determined that you have passed the pre screening process, you will be asked to come into an IRI facility for an in-person screening. Please bring with you your ID, medical history, and list of current medications you are on. This is standard practice across all studies to ensure your safety. Alternatively, if we meet you during a scheduled clinic visit, the screening may occur alongside your current visit or following your visit.
Informed Consent
You will be given an Informed Consent Form (ICF) which details what will happen in the study and allows you to make sure you understand each aspect of the study. After you have understood everything and consent to the study, you will then sign the form. That being said, you may withdraw your consent at any time for any reason.
Physical Exam
Depending on the study, you may receive a physical exam or undergo diagnostic tests – the results of which may be shared with you as a perk of participating.
Administration & Monitoring
When you are administered an investigational product, your health will be monitored closely by the clinical team. To better evaluate the new medicines’ safety and efficacy, you may undergo additional tests as well.
Your safety and health matters to Insight, you will be asked to be an active participant and share your progress honestly and openly during visits. This will help our team ensure your safety throughout the trial.
Compensation
You may be compensated for your participation in a study. More information tailored to the particular study you are interested in can be answered in your pre-screening and screening visits.
Follow up Visits
Most studies require you to come in for follow up visits for the duration of a study. You may be eligible for compensation for your time and travel for each visit.
